Model System: MTB-IGFIR Transgenic Mice
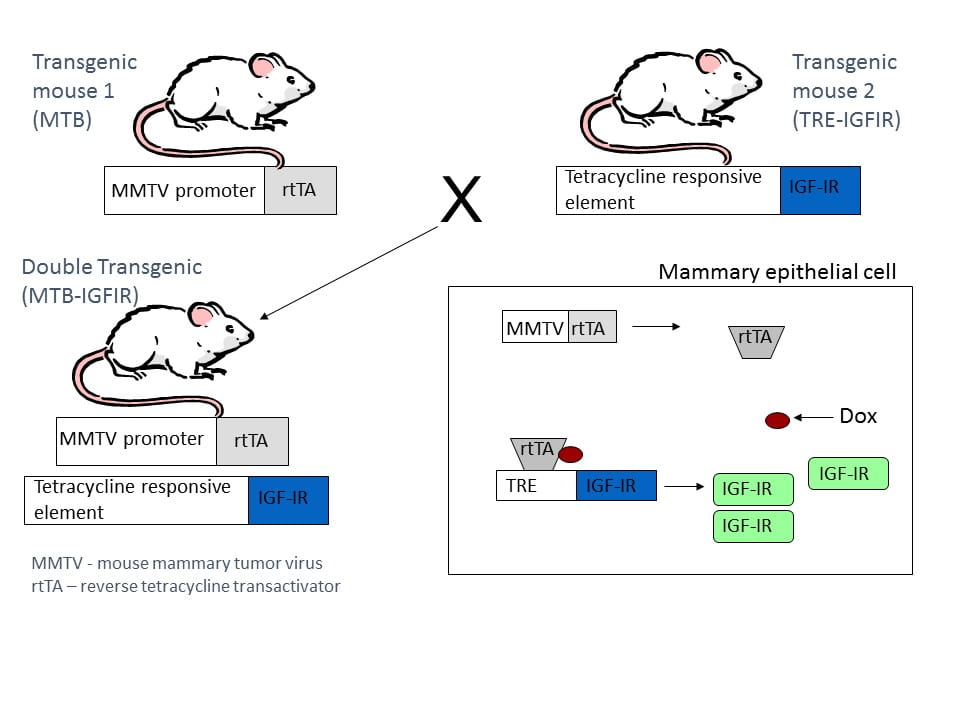
MTB-IGFIR transgenic mice express elevated levels of human type I insulin-like growth factor receptor (IGF-IR) in mammary epithelial cells in a doxycycline inducible manner. In order to generate MTB-IGFIR transgenic mice, two separate transgenic mice must be mated: MTB mice and TRE-IGFIR mice. MTB mice contain the reverse tetracycline transactivator (rtTA) downstream of the mouse mammary tumor virus (MMTV) promoter. The MMTV promoter drives expression of the rtTA only in mammary epithelial cells. Since rtTA has no function in mammalian cells, MTB mice have no phenotype. The MTB mice were generously provided by Dr. Lewis Chodosh. My lab created the TRE-IGFIR mice which have a construct containing human IGF-IR cDNA cloned downstream of a tetracycline response element (TRE). The TRE-IGFIR mice have no phenotype. Breeding MTB mice with TRE-IGFIR mice will generate some offspring containing both transgenes, also know as MTB-IGFIR mice. The MTB-IGFIR mice still have no phenotype until they are fed a tetracycline derivative such as doxycycline in their food or water. Doxycycline alters the conformation of the rtTA thus allowing it to bind to the TRE and drive expression of the IGF-IR transgene (see the figure to the right). This system allows the researcher control of when the transgene is induced, the duration of induction and to some degree, the level of transgene expression (changing the concentration of doxycycline administered to the mice can alter transgene levels).
Cell Lines Derived from MTB-IGFIR Mice
RJ345
These cells were developed from a mammary tumor expressing high levels of IGF-IR. In two dimensional culture these cells have a cubodial appearance and gene expression analysis revealed high levels of epithelial genes such as Cdh1 Cldn3, Cldn4, and Cldn7 and low levels of mesenchymal markers such as Vim, Snai1, Snai2, Zeb1, Zeb2, Twist1 and Twist2. These cells are weakly tumorigenic when injected into the mammary gland of syngeneic FVB mice.
RM11A
These cells were the first cell line we developed from MTB-IGFIR transgenic mice and were derived from a mammary tumor expressing high levels of IGF-IR. These cells have a mesenchymal morphology and express low levels of Cdh1, Cldn3, Cldn4 and Cldn7 and high levels of Zeb1, Zeb2, Twist1 and Twist 2. The phenotype of these cells seems to drift with increasing passage number and culturing these cells in the absence of doxycycline induces and irreversible change in morphology and loss of IGF-IR transgene expression. These cells are tumorigenic in mice and their initial growth is dependent on the presence of doxycycline. We are no longer using these cells due to their instability with increasing passage number.
RJ348
RJ348 cells were developed from a mammary tumor that recurred following doxycycline withdrawal in a MTB-IGFIR transgenic mouse. The tumor from which these cells were derived had a mesenchymal morphology and RJ348 cells maintain this mesenchymal morphology in culture. RJ348 cells express low levels of Cdh1 Cldn3, Cldn4 and Cldn7 high levels of Vim, Snai1, Snai2, Zeb1, Zeb2, Twist1, and Twist2. RJ348 cells are highly tumorigenic when injected into the mammary gland of syngeneic FVB mice. One limitation of the RJ348 cells is they appear extermely resistant to stably expressing genes and shRNA from expression constructs including lentiviral constructs. Transient transfection with siRNA is effective.
RJ423
RJ423 cells were developed from a mammary tumor that recurred following doxycycline withdrawal in a MTB-IGFIR transgenic mouse. The tumor from which these cells were derived had a mesenchymal morphology and RJ423 cells maintain this mesenchymal morphology in culture. RJ423 cells express high levels of mesenchymal genes and only low levels of epithelial genes and like RJ348 cells, RJ423 cells are highly tumorigenic when injected into mammary glands of syngeneic FVB mice. Although we are still characterizing these cells, they do appear to be more amendable to stable transfection.